Ophthalmic Vascular and Neural Research Program
History
With support from the Kruse Endowment Fund and the establishment of the Kruse Centennial Chair in Ophthalmology of Baylor Scott & White Health (BSWH), Dr. Lih Kuo – Regents Professor of Medical Physiology at the Texas A&M Health Science Center with a specialization in microvascular research – was recruited as the Endowed Chair and the Director of the Ophthalmic Vascular Research Program (OVRP) in 2003 through a national search.
The OVRP is the first in the nation and the world to be designated for ophthalmic vascular research in translational medicine. Dr. Robert Rosa (clinician scientist in ophthalmology) and Dr. Travis Hein (basic scientist in microcirculation) joined the OVRP as core members of the team to facilitate retinal disease research. Dr. Kuo moved his laboratories from College Station to the Temple campus in March 2004.
The OVRP has grown significantly in the past 21 years and has received national/international recognition in ophthalmic vascular research and benefited the institution in translational research and education. In 2019, the BSWH eliminated all fundamental research programs on the Temple campus, and the OVRP was then adopted by the Department of Medical Physiology at Texas A&M Health. In 2020, the OVRP moved back to the Texas A&M Medical Campus in Bryan, TX, to continue the mission. Dr. Kuo stepped down as OVRP Director in September 2024, relaying the leadership role to Dr. Hein, Professor of Medical Physiology at Texas A&M Health. To expand the scope of the ophthalmic research, the OVRP was renamed to the Ophthalmic Vascular and Neural Research Program (OVNRP) to include neural retina biology in 2025. Dr. Kayla Bayless, Dr. Gladys Ko, and Dr. Srinath Palakurthi joined the OVNRP in 2025, expanding the faculty expertise in vascular research to neural biology and drug formulation and delivery.
Mission - To discover and deliver next generation therapeutics for vision preservation through innovative vascular and neural research, education, and patient outreach at Texas A&M University.
Vision - To be an international academic leader in advancing cutting-edge research to provide impactful solutions to prevent and treat vascular and neural diseases and complications that threaten sight.
Goals & Achievements
Goals
The long-term goal of the OVNRP is to establish a nationally and internationally recognized ophthalmic research center or Institute at Texas A&M University with interdisciplinary collaborations from basic to translational and clinical research and education to ultimately benefit patient care and improve vision and quality of life.
The short-term (5 years) goals are to:
- Develop retinal disease animal models resembling human pathophysiology;
- Conduct translational research on diabetic retinopathy, hypertensive retinopathy, retinal degeneration, and spaceflight-associated neuro-ocular syndrome; and
- Develop therapies for vision-threatening eye diseases such as diabetic retinopathy, retinitis pigmentosa, macular degeneration, and retinopathy of prematurity.
Achievements
The scientific quality and merit of the OVNRP can be evaluated in five categories:
- Publications
- Research funding
- Scientific review
- International visiting scholars
- Industry consulting
The OVNRP investigators have published more than 60 peer-reviewed premier publications and 17 book chapters on eye-related research.
The OVNRP has been successful in securing grants and funding from multiple foundations, philanthropic donations, as well as the NIH National Eye Institute and NASA.
The OVNRP has also obtained research funding from the Retina Research Foundation for the past 20 consecutive years with several research awards, signifying the scientific quality and merit of the OVNRP. In total, the OVNRP lab and clinical trials have been granted over 20 million dollars throughout the past decade.
Impacts on the Community/Patients
The OVNRP is committed to promoting and advancing medical knowledge in the vision research community and to improving patient care and human health through the education and training of tomorrow’s health leaders. The OVNRP Principal Investigators have trained and mentored more than 12 postdoctoral researchers and 6 PhD students over the past five years. Since 2003, there have been more than 40 MD and PhD trainees under OVNRP supervision.
The OVNRP has been instrumental in research education for many ophthalmology residents at BSWH and medical students at Texas A&M Health. Many OVNRP former trainees are now directly involved in patient care as clinicians and clinician scientists at other institutions. The key members of the OVNRP frequently present and disseminate medical knowledge in vision health and research, and promote vision care locally, nationally, and internationally.
Current Research
- Diabetic Retinopathy: Diabetes (type 1 and type 2) causes progressive damage of small blood vessels and neurons in the retina of the eye that can lead to diabetic retinopathy and vision loss. Diabetic retinopathy is the leading cause of blindness in working-age adults, but effective treatments are limited to the late stage of the retinal disease with only about half of patients experiencing improved vision. Therefore, we are investigating how diabetes impacts the function of small retinal vessels. Our studies demonstrate that early diabetes causes abnormal vasomotor regulation of small retinal arteries and veins, which leads to insufficient blood flow to neural cells for proper function. Because effective therapies are lacking to treat retinal vascular dysfunction in advanced-stage diabetes, our goal is to identify novel destructive proteins in small retinal vessels that can be targeted for therapy to improve blood flow and consequently retinal function in early diabetes before irreversible damage occurs. We are performing imaging of the retina to measure changes in the structure of the neural and vascular retina and the blood flow in the retinal circulation. The techniques below are used to measure structural features of the retinal microcirculation.
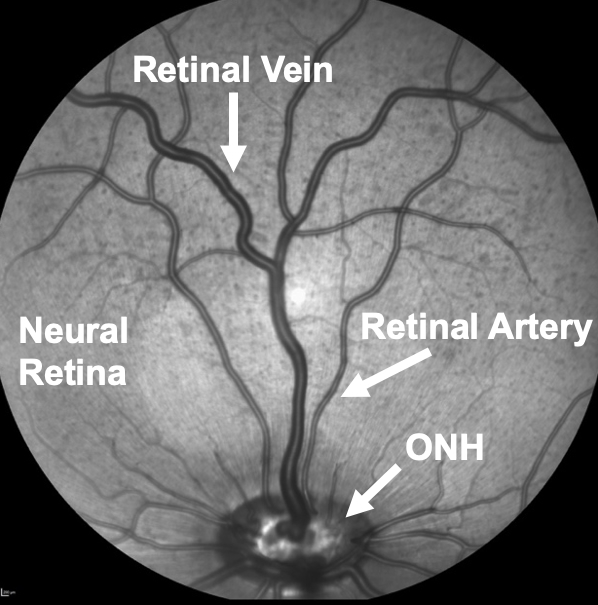 |
 |
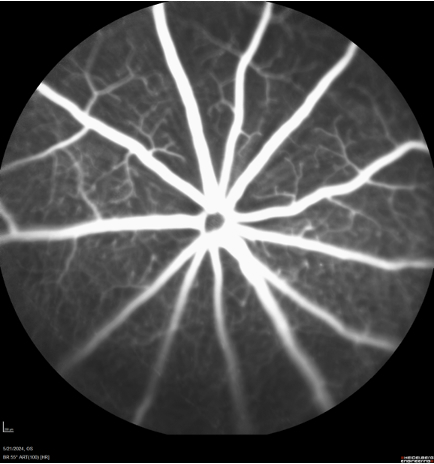 |
Fluorescein Angiography of retinal microcirculation for detecting leakage and abnormal vessel growth.
- Retinal Angiogenesis: We are working to understand how chemical changes that promote eye diseases like retinopathy of prematurity, diabetic retinopathy, and macular degeneration contribute to abnormal or pathological growth of new blood vessels. This new blood vessel growth is associated with retinal damage and vision loss. In our labs, human retinal endothelial cells are grown under conditions that promote new blood vessel growth. We can incorporate factors that are upregulated in disease and monitor changes in the rate of new blood vessel growth, as well as molecular signals that increase or decrease during the process. The image below on the left illustrates how these experiments are carried out in three-dimensional (3D) matrices. The image below on the right is a microscopic image showing an example of blood vessels that we grow and study in a dish.
 |
- Spaceflight-Vision Research: We are working on projects to identify small vessel changes in the eye that may contribute to vision complications in astronauts, known as spaceflight-associated neuro-ocular syndrome (SANS), during long-duration spaceflight missions. The most common ocular change that occurs in spaceflight is optic disc edema (swelling of the inner eye portion of the optic nerve), but the underlying cause remains unclear. Therefore, we are examining whether spaceflight environment factors of microgravity and ionizing radiation alter the function of small vessels in the eye that control edema development, which could promote SANS.
- Hypertensive Retinopathy: Hypertension can contribute to complications in ocular circulation and the development of retinal pathophysiology as well as vision impairment. However, the impact of hemodynamic (blood pressure and blood flow) changes on the retinal circulation and its vasomotor regulation/dysregulation remains unclear. These issues are examined in a mouse model with aortic narrowing to create unequal blood pressure and flow distribution to the right and left eyes. The underlying mechanism of vasomotor dysregulation and associated ocular pathophysiology are studied. The derived information is used for new drug development for treating hypertensive retinopathy.
- Retinal Degenerative Diseases: Stanniocalcins (STC-1/-2) are endogenous polypeptides with anti-inflammatory activity. We proposed that STC-1 exhibits therapeutic activities for inherited and acquired retinal degenerations, including age-related macular degeneration (AMD) and retinitis pigmentosa (RP). Preclinical studies with intravitreal administration of STC-1 in rodent models and a large animal model of inherited retinal degeneration in pigs have demonstrated the rescue of photoreceptor degeneration using multiple testing modalities (electroretinography, photoreceptor gene expression, OCT morphology, and LM/EM histology). Our preclinical studies suggest that STC-1 and STC-2 may be used as a therapeutic tool not only in RP and AMD but also in various forms of genetic and acquired diseases such as hypertensive retinopathy, diabetic retinopathy, and glaucoma by promoting cell-survival gene expression and reducing inflammation and oxidative stress, and thus prevent/delay central vision loss.
- Identification of Novel Peptide: The Ko lab has identified a novel peptide named Peptide Lv (PLv) and has documented its multiple functions. PLv enhances ion channel expression in neurons (L-type voltage-gated calcium channels) and vascular endothelial cells (IKCa; KCa3.1), elicits retinal arteriolar dilation, and promotes retinal angiogenesis. PLv is upregulated in ocular diseases with neovascularization. The expression of Peptide Lv is shown below in the retinal images.
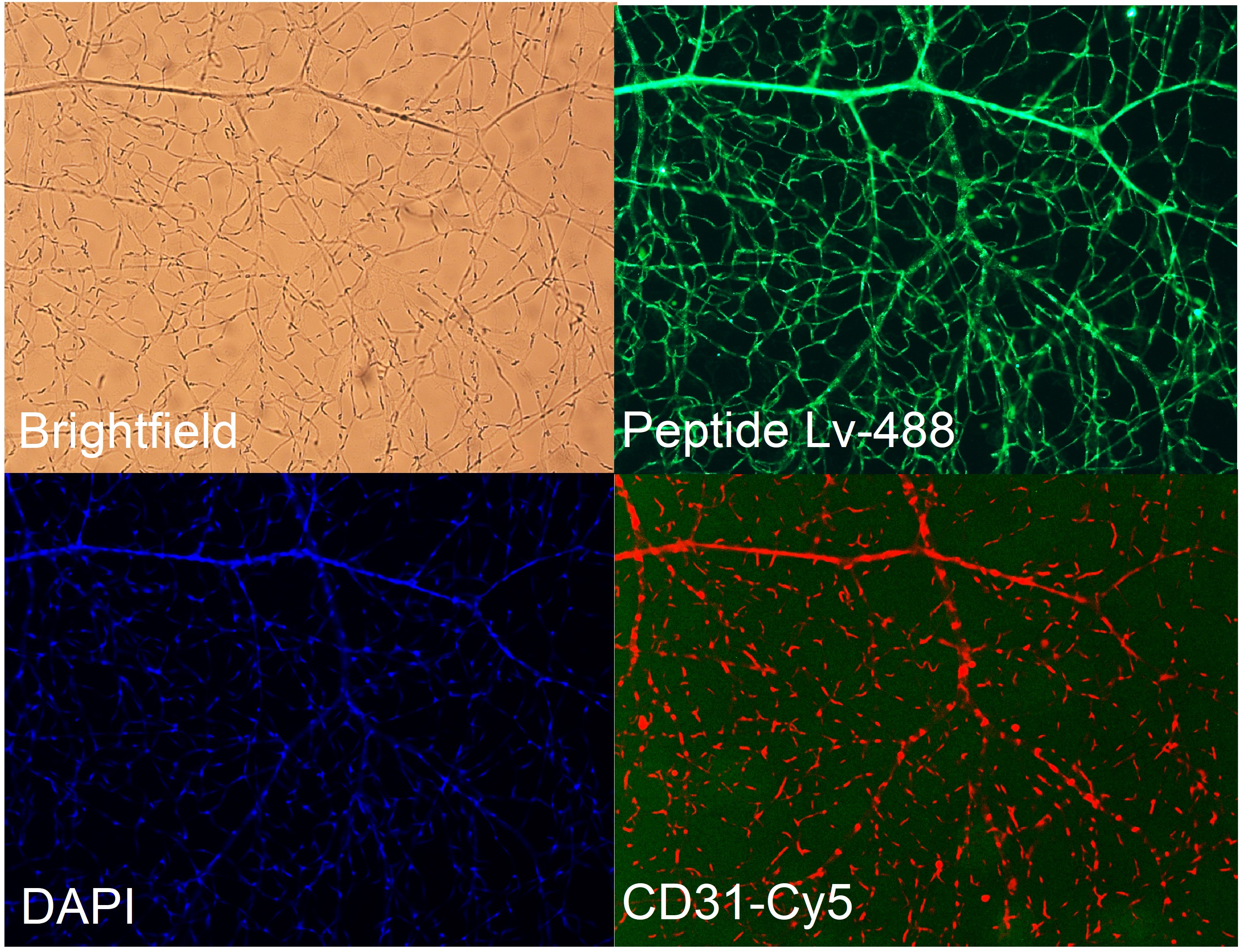 |
- Retinal Development: The Ko lab is studying the impact of preconception paternal alcohol consumption on the offspring’s retinal development and neural function/vision and the mechanisms contributing to the abnormal retinal blood vessel growth and neural function in retinopathy of prematurity. Neural function is tested using electroretinography (ERG) as shown below.
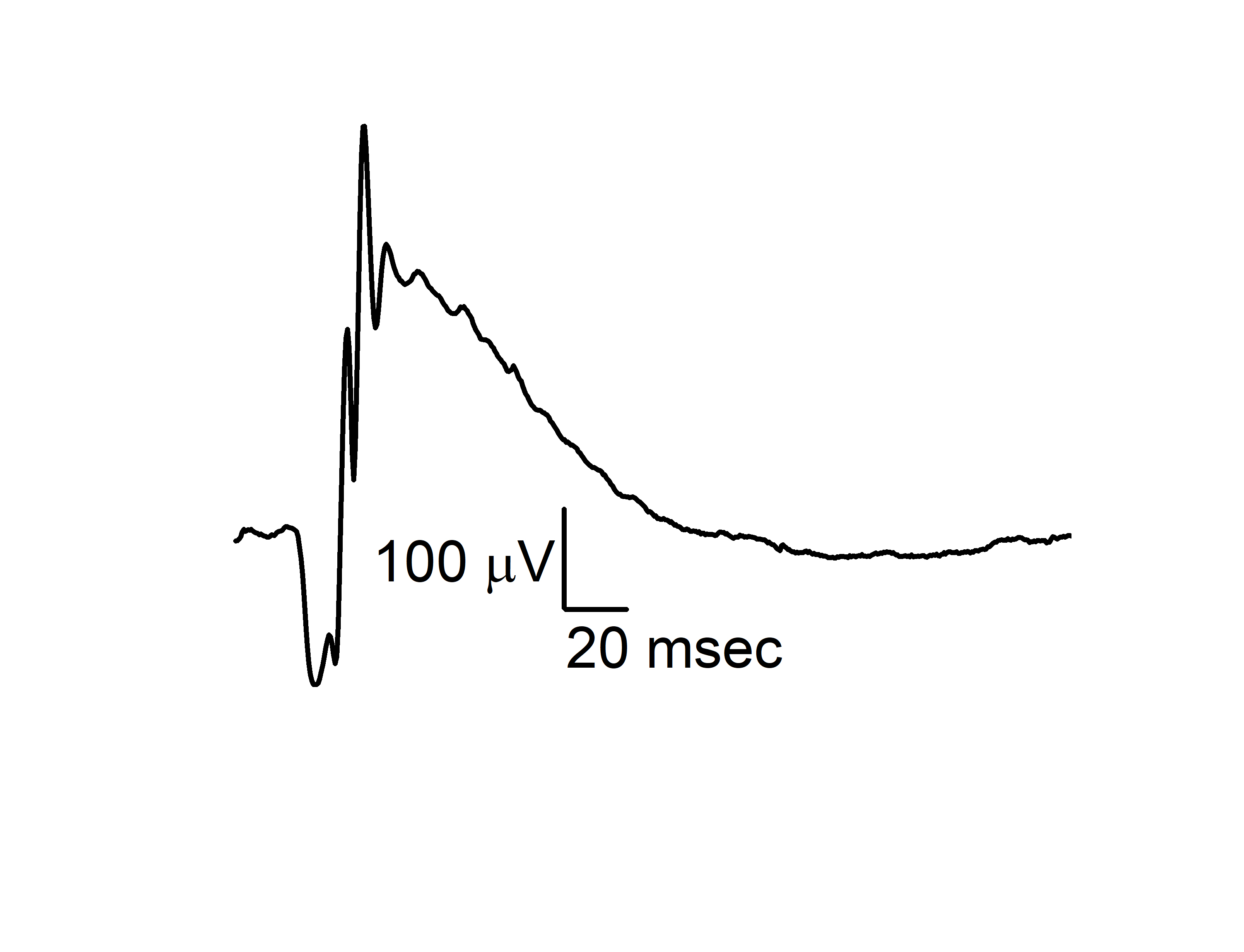 |
Ophthalmic Vascular and Neural Research Program Faculty & Lab Team Members
OVNRP Leadership and Faculty
 |
Travis W. Hein, PhD – Director of the OVNRP Scholars@TAMU Bio |
 |
Lih Kuo, PhD – Founding-Past Director of the OVNRP Scholars@TAMU Bio |
 |
|
 |
Gladys Ko, PhD – Professor Scholars@TAMU Bio |
|
Srinath Palakurthi, PhD – Professor |
|
|
Robert H. Rosa, Jr., MD – Ophthalmologist
|
Ophthalmic Vascular and Neural Team Members
| Colette A. Abbey – Research Technician | Kofi Owusu-Ansah – Graduate Student |
| Joseph Hoppe – Graduate Student | Sushesh Srivatsa Palakurthi – Graduate Student |
| Zahi Hussain – Medical Student | Dylan Pham, PhD – Postdoctoral Research Associate |
| Hyoseon Kim, PhD – Postdoctoral Research Associate | Song Yi Shin, PhD – Postdoctoral Research Associate |
| Azrael Lomenzo – Research Technician | Maharshi Thalla, PhD – Postdoctoral Research Associate |
Recent Peer-Reviewed Publications
- Owusu-Ansah K, Pham DL, Strong B, Liberoni L, Hein TW, Kuo L, Ko ML, Ko GY-P. Peptide Lv contributes to the maintenance of the structural and functional integrity of the neural retina. Invest Ophthalmol Vis Sci. 66:37, 2025. DOI: 10.1167/iovs.66.5.37; PMID: 40423624; PMCID: PMC12124149.
- Owusu-Ansah K, Thomas KN, Cox K, Pham DL, Chen W-L, Ko ML, Golding MC, Ko GY-P. Preconception paternal alcohol consumption elicits postnatal changes in neural retinas of the offspring. Invest Ophthalmol Vis Sci. 66:16, 2025. DOI: 10.1167/iovs.66.2.16. PMID: 39913164; PMCID: PMC11806431.
- Abbey CA, Duran CL, Chen Z, Chen Y, Roy S, Coffell A, Sveeggen TM, Chakraborty S, Wells GB, Chang J, Bayless KJ. Identification of new markers of angiogenic sprouting using transcriptomics: New role for RND3. Arterioscler Thromb Vasc Biol. 44:e145-e167, 2024. DOI: 10.1161/ATVBAHA.123.320599. PMID: 38482696; PMCID: PMC11043006.
- Alrbyawi H, Annaji M, Fasina O, Palakurthi S, Boddu SHS, Hassan N, Tiwari AK, Suryawanshi A, Babu RJ. Rapidly dissolving trans-scleral microneedles for intraocular delivery of cyclosporine A. AAPS PharmSciTech. 25:28, 2024. DOI: 10.1208/s12249-024-02738-5. PMID: 38302687.
- Bayless KJ. Direct involvement of CD8+ T cells in retinal angiogenesis. Arterioscler Thromb Vasc Biol. 43:537-539, 2023. DOI: 10.1161/ATVBAHA.123.319101. PMID: 36947606.
- Pham DL, Niemi A, Blank R, Lomenzo G, Tham J, Ko ML, Ko, G. Y.-P. Peptide Lv promotes trafficking and membrane insertion of KCa3.1 through the MEK1-ERK and PI3K-Akt signaling pathways. Cells. 12:1651, 2023. DOI: 10.3390/cells12121651. PMID: 37371121; PMCID: PMC10296961.
- Rosa RH Jr, Xie W, Zhao M, Tsai SH, Roddy GW, Su MG, Potts LB, Hein TW, Kuo L. Intravitreal administration of stanniocalcin-1 rescues photoreceptor degeneration with reduced oxidative stress and inflammation in a porcine model of retinitis pigmentosa. Am J Ophthalmol. 239:230-243, 2022. DOI: 10.1016/j.ajo.2022.03.014. PMID: 35307380.
- Zhao M, Xie W, Hein TW, Kuo L, Rosa RH Jr. Laser-induced choroidal neovascularization in rats. Methods Mol Biol. 2319:77-85, 2021. DOI: 10.1007/97801-0716-1480-8_9. PMID: 34331245.
- Xie W, Zhao M, Hein TW, Kuo L, Rosa RH Jr. Visualization of retinal blood vessels. Methods Mol Biol. 2319:111-117, 2021. DOI: 10.1007/978-1-0716-1480-8_13. PMID: 34331249.
- Mao Y, Kleinjan ML, Jilishitz I, Swaminathan B, Obinata H, Komarova YA, Bayless KJ, Hla T, Kitajewski JK. CLIC1 and CLIC4 mediate endothelial S1P receptor signaling to facilitate Rac1 and RhoA activity and function. Sci Signal. 14:eabc0425, 2021. DOI: 10.1126/scisignal.abc0425. PMID: 33879602; PMCID: PMC8722429.
- Rosa RH Jr, Xie W, Zhao M, Tsai SH, Roddy GW, Su MG, Potts LB, Hein TW, Kuo L. Intravitreal administration of stanniocalcin-1 rescues photoreceptor degeneration with reduced oxidative stress and inflammation in a porcine model of retinitis pigmentosa. Am J Ophthalmol. 239:230-243, 2022. DOI: 10.1016/j.ajo.2022.03.014. PMID: 35307380.
- Pham DL, Niemi A, Ko ML, Ko GY-P. Peptide Lv augments intermediate-conductance calcium-dependent potassium channels (KCa3.1) in endothelial cells to promote angiogenesis. PLOS ONE. 17:e0276744, 2022. DOI: 10.1371/journal.pone.0276744. PMID: 36282858; PMCID: PMC9595550.
- Uppada S, Zou D, Scott E, Ko G, Pflugfelder S, Kumar MNVR, Ganugula R. Paclitaxel and urolithin A prevent histamine induced neurovascular breakdown alike, in an ex vivo rat eye model. ACS Chem Neurosci. 13:2092-2098, 2022. DOI: 10.1021/acschemneuro.1c00692.
- Renukuntla J, Palakurthi SS, Bolla PK, Clark BA, Boddu SHS, Manda P, Charbe NB, Palakurthi S, Advances in in-vitro bioequivalence testing methods for complex ophthalmic generic products. Int J Pharm. 627:122209, 2022. DOI: 10.1016/j.ijpharm.2022.122209. PMID: 36162609.
- Yu F, Ko ML, Ko GY-P. MicroRNA-150 and its target ETS-domain transcription factor 1 contribute to inflammation in diabetic photoreceptors. J Cell Mol Med. 25:10724-10735, 2021. DOI:10.1111/jcmm.17012. PMID: 34704358; PMCID: PMC8581325.
- Yu F, Ko ML, Ko GY-P. Decreased miR-150 exacerbates neuronal apoptosis in the diabetic retina. Biomedicines. 9:1135, 2021. DOI: 10.3390/biomedicines9091135. PMID: 34572320; PMCID: PMC8469350.
- Hanaguri J, Yokota H, Watanabe M, Yamagami S, Sushiyama A, Kuo L, Nagaoka T. Retinal blood flow dysregulation precedes neural retinal dysfunction in type 2 diabetic mice. Sci Rep. 11:18401, 2021. DOI: 10.1038/s41598-021-97651-3. PMID: 34526573; PMCID: PMC8443656.
- Chen YL, Rosa RH Jr, Kuo L, Hein TW. Hyperglycemia augments endothelin-1-induced constriction of human retinal venules. Transl Vis Sci Technol. 9:1, 2020. DOI: 10.1167/tvst.9.9.1. PMID: 32879758; PMCID: PMC7442874.
- Chen YL, Rosa RH Jr, Kuo L, Hein TW. Contributions of sodium-hydrogen exchanger 1 and mitogen-activated protein kinases to enhanced retinal venular constriction to endothelin-1 in diabetes. Diabetes. 70:2353-2363, 2021. DOI: 10.2337/db20-0889. PMID: 34353852; PMCID: PMC8576499.
- Hanaguri J, Yokota H, Watanabe M, Kuo L, Yamagami S Nagaoka T. Longitudinal stability of retinal blood flow regulation in response to flicker stimulation and systemic hyperoxia in mice assessed with laser speckle flowgraphy. Sci Rep. 10:19796, 2020. DOI: 10.1038/s41598-020-75296-y. PMID: 33188259; PMCID: PMC7666208.
Recent Awards & Honors
- Springer Nature Editor of Distinction Award, Scientific Reports, Springer Nature (Kuo), 2025
- Associate Editor, Atherosclerosis, Thrombosis, and Vascular Biology (Bayless), 2024-present
- Chancellor’s EDGES Fellow, Texas A&M University (Palakurthi), 2024
- Adolphe G. and Josephine Roberts Gueymard Research Project Award, Retina Research Foundation (Kuo), 2023-2024
- R. Kelly Hester Distinguished Teaching Award (Hein), College Level – College of Medicine (Hein), 2023
- Innovation Partners Translational Investment Fund Award, Texas A&M (Kuo, Hein), 2022-2023
- Regents Professorship Award, Texas A&M University System (Kuo), 2019
- Association of Former Students of Texas A&M Distinguished Teaching Award – College Level – College of Medicine (Hein), 2017