Joint Microscopy Laboratory
About the Laboratory
The mission of the Joint Microscopy Laboratory (JML) is to encourage researchers to explore advanced single-molecule fluorescence (SMF) methods and SMF imaging modalities and to incorporate them into their research programs.
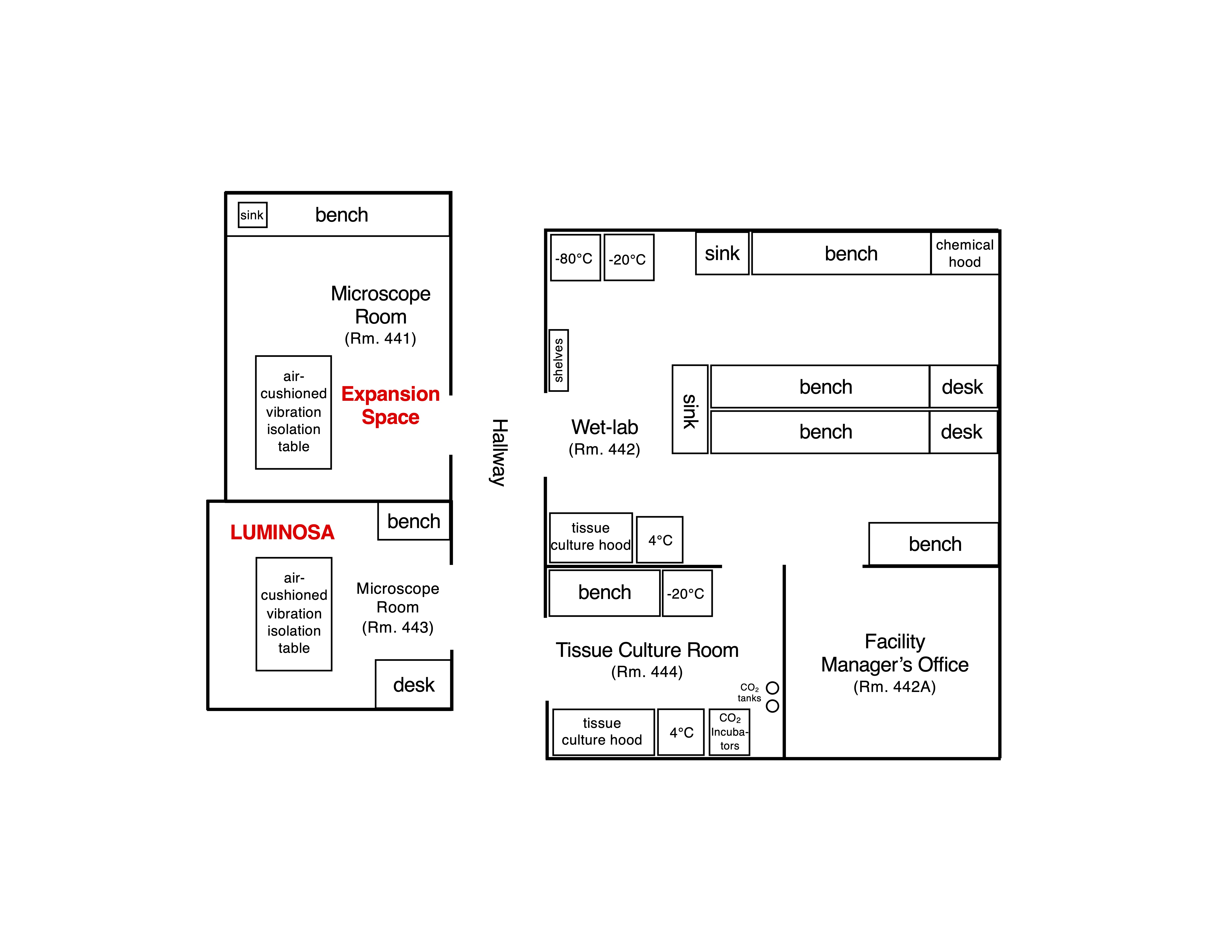
The JML provides technical expertise to support the utilization of facility instruments for the faculty, students, and staff of Texas A&M University and neighboring institutions. The JML includes two microscopy rooms and supporting facilities.
Technical staff is available to train and assist with the design, implementation, and analysis of experiments as well as to assist in troubleshooting.
Training
In-house equipment demonstrations will be provided to illustrate the capabilities and potential applications of facility instrumentation. Workshops for introducing equipment to potential user communities will be organized occasionally. Training sessions will be organized by the Facility Manager on an as-needed basis.
The normal procedure for initiating and continuing the use of JML instrumentation is as follows:
- Interested investigators (students, postdocs, etc.) should contact the Facility Manager and/or the Facility Director regarding the feasibility and design of their proposed experiments. While initial verbal or electronic discussions are encouraged, all projects must be submitted though the Initial Inquiry Form page.
- A preliminary set of experiments will be scheduled and conducted.
- The investigator and the Facility Manager (and, if warranted, the Facility Director) will then consult to plan the costs and time frame needed to accomplish the goals of the proposed experiments. The default assumption is that all equipment use will be ‘assisted’, that is, the Facility Manager will run the equipment for all experiments based on the research needs of the investigator.
An investigator planning an extensive set of experiments or long-term projects should consider becoming a certified user for the instrument(s) needed. Certified users will be able to independently use the instrument(s) on which they are qualified (’unassisted’ use). ‘Unassisted’ use has a lower hourly rate and is available 24/7. There is no set time or training period required for instrument certification. Rather, the investigator’s skill set and a technical understanding of the instrumentation and features needed for the proposed experiments will be the determinants for certification. The Facility Manager is the only person who can certify users, with appeal to the Facility Director. This policy is required to ensure that the care, calibration, and maintenance of the instruments continues to meet the highest standards.
NO TRAINING ON MICROSCOPE SYSTEMS WILL BE PROVIDED WITHOUT PRIOR LASER SAFETY TRAINING CERTIFICATION. Laser safety training is offered by Texas A&M Environmental Health and Safety, Radiological Safety (https://ehsdtraining.tamu.edu). A copy of the Laser Safety Training completion certificate must be emailed to the Facility Manager for record-keeping.
Reservations
Instrument time can be scheduled through Microsoft Teams for the calendar. Once users have completed training, they will be given access to the calendar. Time should be reserved by creating an ‘event’ and including the name of the user and PI. Instrument use (including after-hours and weekend use) can be scheduled up to 3 weeks in advance (4 weeks for Major Users). Instrument availability will be on a first-come, first-served basis with up to 60% of the time being allocated to Major Users. Double booking of a time slot is not allowed. Researchers should estimate the scheduled time as accurately as possible. Assisted instrument use requires at least 48 hours’ notice, unless arranged directly with the Facility Manager.
Users MUST sign in and out of the instrument by using the log sheets present in each room at the start and end of use time. The instrument logs and the online scheduling calendars will be used to determine billable hours. Both are required.
Schedules are subject to rearrangement by the Facility Director and Facility Manager, if necessary. An effort will be made to contact users if rearrangements are needed. Scheduled service will be blocked out ahead of time, but emergency/unscheduled service may occasionally be needed.
A user may cancel scheduled time without charge if the cancellation is made at least 48 hours in advance of the scheduled time. If someone else uses time cancelled within 48 hours of start, the original user will not be charged.
RATES
|
Instrument |
Rate/Hour |
Microsoft Teams Calendar Name |
|
Luminosa Confocal Microscope Internal users (assisted use) Internal users (unassisted use)* External users – Academic (assisted use) External users – Industry (assisted use)
|
$25 $12 $70 $140 |
LUMINOSA_Calendar |
|
Fluoromax Plus Spectro-Fluorometer Internal users (assisted use) Internal users (unassisted use)* |
$15 $10
|
|
*Requires approval of facility staff
Facility Map
Biohazard Safety
The JML laboratory rooms meet the criteria for Biosafety Level-2 (BSL-2). Under no circumstances are BSL-3, BSL-4 agents/pathogens or radioactive material allowed in the facility at any time.
BEFORE any BSL-1 or BSL-2 material is brought into the JML facility, the applicable microscope and support rooms must be added to the Principal Investigator’s approved TAMU Institutional Biosafety Committee (IBC) permit (in Section F, Agent Use and Storage Locations). The investigator should provide confirmation that the IBC permit lists the JML rooms (e.g., the approved permit) and verification of user training (e.g., the training certificates) to the Facility Manager.
Where feasible, the manipulation of biohazardous samples before imaging should be done in the user’s lab. However, recognizing that sample stability and timing may require on site preparation, the JML facility includes wet-lab and biosafety cabinet space. When possible, seal culture dishes or other sample chambers (e.g., with parafilm or via other means). Samples must be placed in secondary containment before transporting to the JML facility. All reasonable precautions must be made to prevent spills on or near the microscope equipment. The microscopes are very sensitive to cleaning agents. ANY DAMAGE TO THE MICROSCOPE RESULTING FROM SPILLED SAMPLES, INCLUDING NECESSARY DISINFECTION WITH HARSH CHEMICALS, WILL BE THE RESPONSIBILITY OF THE USER AND PI. The instruments, associated stages and incubation/sample chambers, and any other surfaces should be disinfected with 70% ethanol. Solid biohazardous wastes must be disposed of in the provided biohazard waste bags, which will be autoclaved. If a user’s samples require disinfection by an alternate disinfectant, this will be used on all non-instrument surfaces. The user will need to provide such alternate disinfectants. Bleach-containing solutions or materials should NOT be autoclaved.
In the event of a spill, spill kits can be found in each laboratory. Assess the personnel involved first. If any biohazardous material gets in your eyes, flush your eyes at the nearest eyewash immediately (in Room 442). Remove any contaminated clothing or personal protective equipment (PPE) and wash any exposed areas of skin with soap and water. Put on clean clothing (if necessary) and fresh PPE (i.e., lab coat, gloves, and eye protection) before cleaning the spill. NOTIFY DR. SAU or DR. MUSSER IMMEDIATELY. Cover the spill with paper towels. Consider that the spill may extend farther than you think and may have contaminated other surfaces nearby. Apply agent-appropriate disinfectant on the spill area, working from just outside the margins of the spill towards the center. Use only 70% ethanol on the microscope and optical table. If reasonable, allow Dr. Sau to decontaminate the microscopes and any other sensitive equipment. Allow for sufficient disinfectant contact time. Note: the minimum contact time may vary, depending on the disinfectant(s) in use and the agent(s) being treated. Pick up any broken glass using forceps, tongs, or a broom and dustpan. NEVER pick up glass with your bare hands. Ensure that glass is decontaminated before disposing in broken glass or sharps container. Dispose of bleach-soaked paper towels (or other absorbent materials) as non-biohazardous waste. Bleach containing solutions or materials should NOT be autoclaved. Other solid biohazardous waste must be collected in a biohazard waste bag and autoclaved. Repeat the disinfection of the spill site to ensure that the area is thoroughly cleaned and disinfected. Immediately report any spill of risk group 2 materials outside the biosafety cabinet, any spill of risk group 1 material in excess of 25 ml, and any spill of recombinantly modified risk-group 1 material to Dr. Abhishek Sau and to the Office of Biosafety (calling 979-862-4549 or e-mail biosafety@tamu.edu). For after-hours spill emergencies, please call the Communications Center at 979-845-4311 for assistance.
Publications Info
Use of the JML facility requires acknowledgement of the Attribution Policy described below, which follows the guidelines promulgated by Angeletti and coworkers (1999, FASEB J. 13:595-601). These guidelines state that “Intellectual interactions between resource and research scientists are essential to the success of each project. When this success results in publication, a citation in the acknowledgments section of a manuscript may be appropriate for routine analysis. However, contributions from resource scientists that involve novel resource laboratory work and insight, experimental design, or advanced data analysis that make a publication possible or significantly enhance its value require co-authorship as the appropriate acknowledgment.”
The JML Attribution Policy requires the following:
- Acknowledgement of the instrument used and the grant supporting acquisition of the instrument. Staff assistance regarding instrument training and proper usage, standard data analysis, and general advice on sample preparation requires acknowledgment of the individual’s contribution (by name) in the acknowledgements section of the manuscript.
- Authorship is expected when a staff individual makes substantial intellectual and/or experimental contribution towards a project.
For publications containing data from the Luminosa confocal microscope, please include the following in the acknowledgment section:
"The authors acknowledge the assistance of the Joint Microscopy Laboratory in the Texas A&M University School of Medicine and the NIH for instrument funding support of the Luminosa confocal microscope (S10 OD032208)."
For publications containing data from the Fluoromax Plus Spectro-Fluorometer, please include the following in the acknowledgment section:
"The authors acknowledge the assistance of the Joint Microscopy Laboratory in the Texas A&M University Naresh K. Vashisht of Medicine and the NIH for instrument funding support of the Fluoromax Plus Spectro-Fluorometer (R01 GM126190-05S1)."
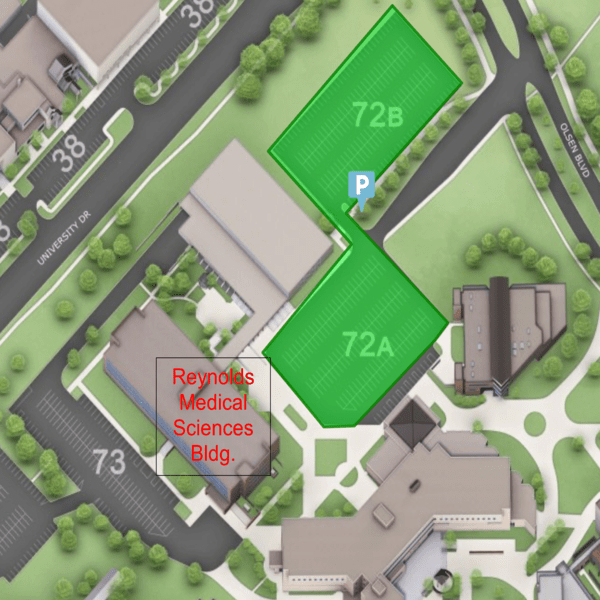
Location and Parking
The JML Laborary is located on the 4th floor of the Reynolds Medical Sciences Bldg.
Public Pay parking is available in lot 72 (highlighted in green).