Adam Nock, PhD

Assistant Professor
Contact
Microbial Pathogenesis & Immunology
8447 John Sharp Parkway
Medical Research and Education Building I - 3102
Bryan,
TX
77807-3260
adamnock@tamu.edu
Phone: 979.436.0354
www.rickettsialab.org
Biography
- Rocky Mountain spotted fever is arguably the most deadly tickborne diesase, with historical mortality rates in some locations exceeding 65%. This disease is caused by Rickettsia rickettsii, an obligate intracellular bacteria that is vectored by multiple tick species relevant to human health. The disease has been endemic to Western Montana into Idaho, and in Brazil. However today there is higher prevalence of the disease in the American Southwest where R. rickettsii is vectored by the brown dog tick, and the disease occurs throughout the continental United States. When introduced into the host via tick bite, R. rickettsii invades host cells, rapidly escapes into the cytosol, and establishes a replicative niche through the modification of host cell metabolism using secreted effectors. Most famously, rickettsia of the spotted fever group are typically capable of coopting host-cell actin to achieve motility and spread from one host cell to another, a feature critical for virulence.
- During my postdoctoral training in Ted Hackstadt’s laboratory, we discovered a rickettsial protein, RoaM (regulator of actin-based motility), that negatively regulates the production of actin tails and the expression of several hypothetical proteins, including various secreted effectors. These findings are particularly exciting because little is known about how R. rickettsii senses and responds to changing environments as the bacteria transitions through its zoonotic cycle from arthropod vector, to vertebrate host, and back again. This is in part because rickettsia possess a reduced genome, a typical evolutionary adaptation found in organisms with a parasitic life cycle. Even considering the reduced size of the genome, rickettsia possess a smaller proportion of bioinformatically identifiable regulators compared to model bacteria such as Escherichia coli or Pseudomonas aeruginosa. Further, we found that roaM is strongly selected against in cell culture and loss or truncation of roaM results in hyper-spreading mutants. Genomic sequencing of various rickettsia suggests that many experiments have inadvertently been performed with hyper-spreading strains and some observed phenotypes in the literature have been misattributed due to the passage of rickettsia strains tending to quickly result in the generation of hyper-spreaders.
- Determining how R. rickettsii successfully navigates the transitions between ticks and vertebrate hosts by sensing and responding to environmental changes is a central theme in my laboratory. To investigate this topic, we use a combination of bacterial genetics, microscopy, and biochemistry. Specific questions include building on what we know about RoaM so far and determining the mechanism behind the repression of actin-based motility. We will also investigate the functions are of RoaM-regulated effectors on host cells. Finally we will expand on our previous work developing genetic tools for rickettsia. Because rickettsia are obligate intracellular bacteria with little known about the details of their transcription and translation, they have long been considered genetically intractable. We were successfully able to generate a recombinant knockout for roaM, developed an anhydrotetracycline-inducible expression system, and CRISPRi plasmid for R. rickettsii. Fine tuning and building on these systems to make them more reliable and add new facets will greatly contribute to the ability of laboratories to study rickettsial biology.
Education and Training
- Rutgers University, BA (Dr. Tom Montville), 2005
- Rockefeller University, Research Assistant (Drs Sohail Malik and Robert Roeder), 2005-11
- University of Vermont, PhD (Dr. Matthew Wargo), 2011-18
- Rocky Mountain Laboratories (NIAID), Postdoctoral Training (Dr. Ted Hackstadt), 2018-24
Representative Publications
- Development of inducible promoter and CRISPRi plasmids functional in Rickettsia rickettsii. J Bacteriol. 2024 Oct 24;206(10):e0036724. doi: 10.1128/jb.00367-24. Epub 2024 Sep 30. PubMed PMID: 39347571; PubMed Central PMCID: PMC11500500.
- Completed genomes for Rickettsia rickettsii isolated from ticks and quality controlled for motility phenotype. Microbiol Resour Announc. 2023 Oct 19;12(10):e0036223. doi: 10.1128/MRA.00362-23. Epub 2023 Sep 1. PubMed PMID: 37655895; PubMed Central PMCID: PMC10586118.
- Identification of an autotransporter peptidase of Rickettsia rickettsii responsible for maturation of surface exposed autotransporters. PLoS Pathog. 2023 Jul;19(7):e1011527. doi: 10.1371/journal.ppat.1011527. eCollection 2023 Jul. PubMed PMID: 37523399; PubMed Central PMCID: PMC10414592.
-
Regulator of Actin-Based Motility (RoaM) Downregulates Actin Tail Formation by Rickettsia rickettsii and Is Negatively Selected in Mammalian Cell Culture. mBio. 2022 Apr 26;13(2):e0035322. doi: 10.1128/mbio.00353-22. Epub 2022 Mar 14. PubMed PMID: 35285700; PubMed Central PMCID: PMC9040884.
- Characterizing species interactions that contribute to biofilm formation in a multispecies model of a potable water bacterial community. Microbiology (Reading). 2020 Jan;166(1):34-43. doi: 10.1099/mic.0.000849. Epub 2019 Oct 4. PubMed PMID: 31585061; PubMed Central PMCID: PMC7137775.
- Choline Catabolism in Burkholderia thailandensis Is Regulated by Multiple Glutamine Amidotransferase 1-Containing AraC Family Transcriptional Regulators. J Bacteriol. 2016 Sep 15;198(18):2503-14. doi: 10.1128/JB.00372-16. Print 2016 Sep 15. PubMed PMID: 27381916; PubMed Central PMCID: PMC4999938.
- Characterization of the GbdR regulon in Pseudomonas aeruginosa. J Bacteriol. 2014 Jan;196(1):7-15. doi: 10.1128/JB.01055-13. Epub 2013 Oct 4. PubMed PMID: 24097953; PubMed Central PMCID: PMC3911141.
-
SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol. 2013 Mar;195(6):1214-25. doi: 10.1128/JB.02181-12. Epub 2013 Jan 4. PubMed PMID: 23292781; PubMed Central PMCID: PMC3592010.
- Mediator-regulated transcription through the +1 nucleosome. Mol Cell. 2012 Dec 28;48(6):837-48. doi: 10.1016/j.molcel.2012.10.009. Epub 2012 Nov 15. PubMed PMID: 23159738; PubMed Central PMCID: PMC3534924.
- Identification of DNA-dependent protein kinase as a cofactor for the forkhead transcription factor FoxA2. J Biol Chem. 2009 Jul 24;284(30):19915-26. doi: 10.1074/jbc.M109.016295. Epub 2009 May 28. PubMed PMID: 19478084; PubMed Central PMCID: PMC2740417.
- Inhibition of Bacillus anthracis and potential surrogate bacilli growth from spore inocula by nisin and other antimicrobial peptides. J Food Prot. 2006 Oct;69(10):2529-33. doi: 10.4315/0362-028x-69.10.2529. PubMed PMID: 17066940.
Research Images

Female American dog tick laying eggs.
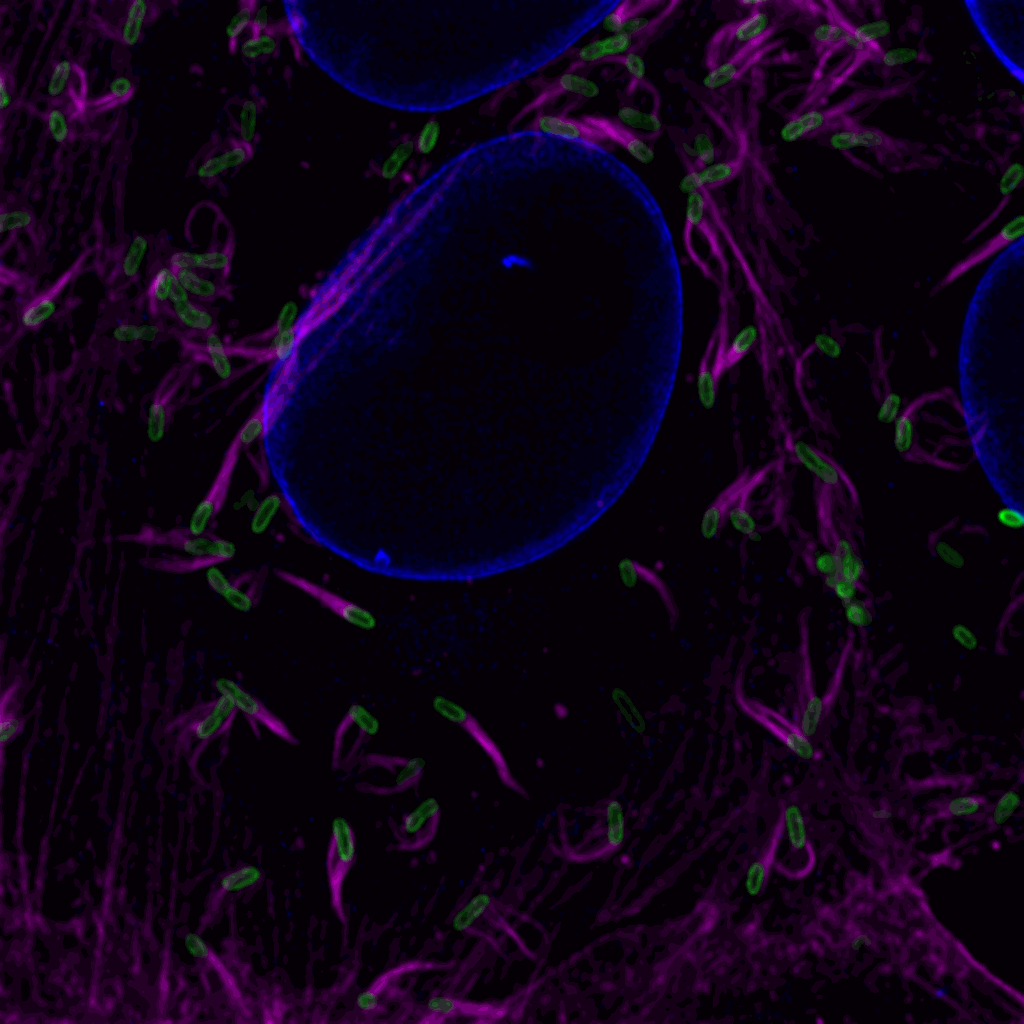
Confocal image of R. rickettsii Sheila Smith (green) producing actin tails (magenta) in Vero host cells (host cell nuclear envelope, blue).