Hui-Wen Lo

Department Head, Cell Biology and Genetics
Professor
Jean and Tom McMullin Endowed Professor
Contact
Cell Biology and Genetics
8447 John Sharp Parkway
Bryan,
TX
77801
hui-wen.lo@tamu.edu
Biography
Dr. Hui-Wen Lo is a basic and translational cancer biologist with more than 30 years of experience conducting research and scientific discoveries, leading to two patents and one clinical trial. As a nationally and internationally recognized cancer scientist, Dr. Lo has been invited to speak and participate in many grant review panels for the NIH, DoD, and agencies worldwide and to serve on several journal editorial boards. Over the past 20 years, her laboratory has been continuously funded by close to $20 million of NIH and DoD grants and from private foundations. Throughout her academic career, Dr. Lo has had many leadership roles at the departmental and institutional levels. In addition to being a seasoned cancer researcher and a leader, Dr. Lo is committed to mentoring the next generation of scientists, including graduate students, medical students, physician scientists, and junior faculty.
Dr. Lo received her PhD in Biochemistry and Molecular Biology from McGovern Medical School at UTHealth Houston. Her PhD dissertation was titled "Signaling pathways in the transcriptional and post-translational regulation of the human glutathione S-transferase P1 gene in human glioblastoma."
Before her PhD studies, Dr. Lo obtained a Master of Arts degree in Nutritional Sciences from the University of Texas at Austin and a Master of Science degree in Biomedical Sciences from UTHealth Houston. Her MS thesis was focused on molecular cloning and structural characterization of hGSTP1*C, an allelic human pi class glutathione S-transferase gene variant from human glioblastoma. Dr. Lo conducted her NCI- and ACS-funded postdoctoral fellowship, followed by an Instructor position at the University of Texas MD Anderson Cancer Center, where she investigated the impact of novel EGFR signaling on breast cancer.
Dr. Lo started her independent academic career at Duke University School of Medicine and NCI-designated Duke University Comprehensive Cancer Center in 2006 as a tenure-track assistant professor. While at Duke, Dr. Lo excelled not only in publications but also in her extramural funding from the NCI, DoD, and private foundations, with more than $3 million in funding. She was promoted to a tenure-track associate professor in 2012 and continued to make breakthrough discoveries. In 2014, Dr. Lo was recruited to Wake Forest School of Medicine and NCI-designated Wake Forest Comprehensive Cancer Center as a tenured associate professor. Four years later, in 2018, she was promoted to professor with tenure. At Wake Forest, Dr. Lo demonstrated herself as an outstanding educator, mentor, researcher, and leader. She has trained many graduate students, postdoctoral fellows, and physician scientists. Some of these individuals successfully obtained NIH funding, faculty positions, and industry positions. In addition to her roles as a mentor and principal investigator, she served as an executive leader in the NCI-designated Wake Forest Comprehensive Cancer Center. As the Associate Director for Shared Resources Management and the Associate Director for Basic Sciences, Dr. Lo helped to bolster the research programs and obtain successful renewal of the NCI Cancer Center Support Grant (CCSG) for the Wake Forest Comprehensive Cancer Center.
In 2022, Dr. Lo joined the Vivian L. Smith Department of Neurosurgery in the McGovern Medical School at UTHealth Houston as a tenured professor. She led the Metastatic Brain Tumor Research Program as the inaugural director. In her role of Vice Chair for Research, Dr. Lo led the research enterprise of the large Neurosurgery department with more than 50 faculty including 20 PhD faculty, which ranked number 5 in NIH funding in 2024 by Blue Ridge Biomedical Institute. She expanded her research program in primary and metastatic CNS malignancies, as well as metastatic breast cancer. Her research program continues to be highly collaborative and multidimensional, with high potential for clinical translation.
Most recently, in 2025, Dr. Lo joined the Texas A&M University Naresh K. Vashisht College of Medicine as Jean and Tom McMullin Endowed Professor and the Head of the Department of Cell Biology and Genetics. She brings her scientific expertise in breast cancer, brain metastasis, and glioblastoma, a well-funded research program, and excellent administrative and leadership skills to her new position. She also secured a joint professor appointment with the Institute of Biosciences and Technology at the Texas Medical Center in Houston. She is actively recruiting graduate students and scientists to her laboratory at Texas A&M Health Science Center, as well as tenure-track assistant professors to the Department of Cell Biology and Genetics.
Education and Training
- University of Texas at Austin, Austin, Texas, M.A. in Nutritional Sciences, 1987-1990
- The University of Texas Health Science Center at Houston, Houston, Texas, M.S. in Biomedical Sciences, 1992-1994
- The University of Texas Health Science Center at Houston, Houston, Texas, Ph.D. in Biochemistry and Molecular Biology, 2000-2002
- University of Texas at MD Anderson Cancer Center, Houston, Texas, Postdoctoral Fellow in Cancer Research, 2002-2004
Research Interests
- Dr. Lo's research interests are primarily in molecular and cell biology that underlies tumor growth, tumor progression, brain metastasis, and cancer drug discovery and development. The Lo Laboratory has focused on three cancer types: breast cancer, breast cancer brain metastasis, and glioblastoma (GBM, the most common and deadliest brain malignancy in adults). Aberrant cell signaling is a major hallmark of almost all types of cancer. Thus, the majority of targeted therapies have been directed against components of important signaling pathways including protein kinases and transcription factors. The lab has investigated several of these pathways, including those mediated by EGFR and HER2, as well as the effectors downstream of both kinases, such as AKT, HSF1, and STAT3. Another major direction of the lab is to investigate molecular mechanisms underlying breast cancer brain metastasis, the end-stage of breast cancer progression that does not have an effective treatment. The lab is particularly interested in investigating transcription factors, kinases and non-coding microRNAs for their roles in driving breast cancer metastasis to the brain and other organs. In addition to breast cancer, the lab is actively investigating glioblastoma, working to understand the role of a novel tumor suppressor, TUSC2, in GBM. The lab is also actively testing novel combination therapies for GBM. The Lo lab is actively involved in developing novel compounds that target important mediators of breast cancer metastasis and glioblastoma progression. Overall, the research conducted in the Lo Laboratory is multidimensional and highly collaborative and translational. She is actively recruiting graduate students and scientists to her laboratories at Texas A&M Health Science Center at both Bryan and Houston campuses.
Representative Publications
Current Projects
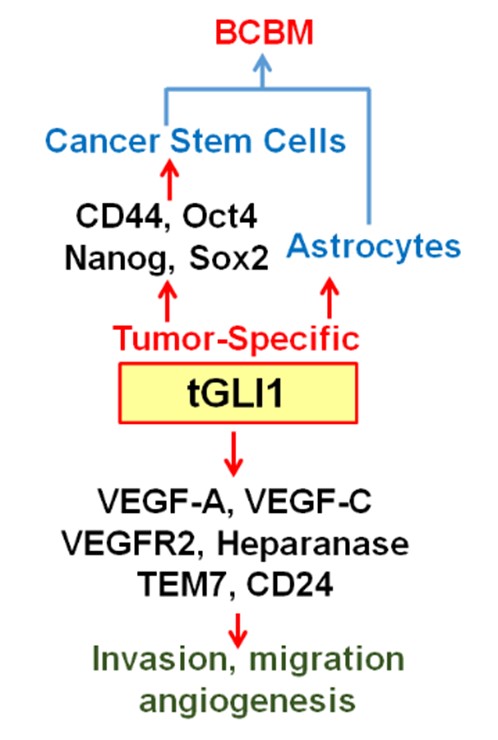
(1) Characterization of tGLI1 and miRNA-1290 for their roles in Breast Cancer Brain Metastasis (BCBM)
The goal of this project is to determine the role of tGLI1 (truncated glioma-associated oncogene homolog 1) in BCBM. Our laboratory discovered tGLI1 as an alternatively spliced GLI1 that lacks entire exon 3 and part of exon 4, but retains the ability to undergo nuclear transport and respond to sonic hedgehog-smoothened signaling. In addition to activating known GLI1 target genes, tGLI1 gains the ability to activate genes not regulated by GLI1, leading to increased migration, invasion and angiogenesis. Our mouse studies indicated that tGLI1 promotes breast cancer preferential metastasis to the brain (and conversely, tGLI1 knockdown selectively suppressed BCBM. tGLI1 protein is overexpressed in lymph node metastases and BCBM samples. In elucidating how tGLI1 promotes BCBM, we found that exosomes secreted from tGLI1-high cancer cells strongly activated astrocytes, the most abundant brain cells known to promote tumor growth when activated, and that tGLI1-high cancer cells had an increased ability to interact with and activate astrocytes in vitro and in vivo. Since microRNAs can be loaded into exosomes and circulating tumor exosomal miRNAs can prime distant organs for organ-specific metastasis, we conducted an exosomal miRNA microarray followed by validations and found that tGLI1-high cancer cells secreted high levels of exosomal miR-1290 and miR-1246 in vitro and in vivo. Whether miR-1290 and miR-1246 play any role in brain metastasis of any cancer or astrocytes is unknown. We observed that miR-1290/1246 inhibited expression of two transcription repressors (FOXA2 and SOX9), which leads to secretion of ciliary neurotropic factor (CNTF) to activate astrocytes and stimulate cancer cells. Ongoing projects in the Lo Lab are to identify novel mechanisms for BCBM.
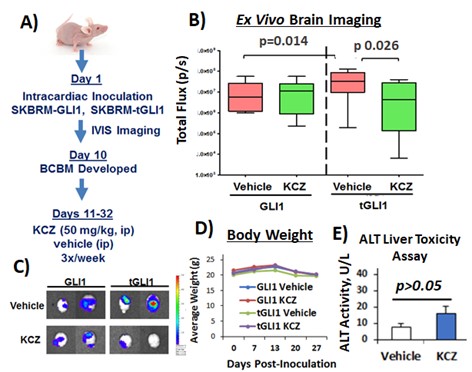
(2) Development of Novel tGLI1 Inhibitors for the Treatment of BCBM and Glioblastoma (GBM)
Given the tumor-specific expression of tGLI1, its high expression in BCBM and GBM sample, we hypothesized that pharmacological inhibitors of tGLI1 can effectively inhibit BCBM and GBM. Currently, there are no available tGLI1 inhibitors. Molecular structure for tGLI1 (or GLI1) is not available to allow for in silico identification of potential tGLI1 inhibitors. To initiate the effort in identifying tGLI1-targeted small molecules, we conducted cell-based screens followed by preclinical validations, and we identified an FDA-approved orally active antifungal KCZ to selectively target tGLI1-driven BCBM and effectively penetrate the BBB/BTB in mice without liver toxicities. These results have led to an active Phase 0 trial for BCBM and GBM (NCT03796273). We are continuing our efforts in developing effective and non-toxic tGLI1 inhibitors for the treatment of BCBM and GBM.
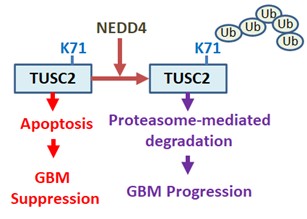
(3) Novel Tumor Suppressor TUSC2 in GBM Development and Progression.
Molecular mechanisms for progression of GBM are still not completely understood. The goal of this application is to fill this knowledge gap by examining the role that tumor suppressor candidate 2 (TUSC2; also known as FUS1) plays in GBM progression. TUSC2 was initially identified as a candidate tumor suppressor gene that is deleted in some lung cancer. TUSC2's tumor suppressive effects in lung cancer are attributed to inducing cell cycle arrest and apoptosis although its exact functionality is unknown. In GBM, TUSC2 gene deletion is very rare (1/580 cases). Expression pattern of TUSC2 in GBM versus its cells of origin has never been examined. Whether TUSC2 plays any functional role in GBM is still mostly unknown. We recently reported an important tumor suppressive function of TUSC2 and its degradation by NEDD4 E3 ligase in GBM. CRISPR/Cas9-mediated TUSC2-knockout promotes in vivo progression of TUSC2(+) GBM xenografts. TUSC2 expression restoration inhibits TUSC2(-) PD-GSCs (patient-derived glioma stem cells) in vitro and in vivo. TUSC2 protein is expressed in normal brain, but is lost or weakly expressed in the majority of GBM specimens and cell lines, and PD-GSCs; however, TUSC2 mRNA is expressed in both normal brain and GBMs. In contrast, both TUSC2 mRNA and protein are lost in lung cancer, but are highly expressed in normal lung tissues. TUSC2 protein is destabilized in GBM compared to astrocytes. TUSC2 interacts with NEDD4 E3 ubiquitin ligase, leading to polyubiquitination at K71 and proteasome-mediated degradation. TUSC2 polyubiquitination has not been reported. Ongoing projects in the Lo Lab are aimed at investigating the regulation of TUSC2 and elucidating TUSC2 functions in GBM.
Lab Members

Mariana Najjar, PharmD, M.S.
PhD Student
Phi Long Tran, Ph.D.
Postdoctoral Fellow
Joshua Cha, B.S.
MS Student
Elissa Bloom, B.S.
PhD Student
Ankush Chandra, MD, MS
PGY5 Neurosurgery Resident
Carly Hsu, B.S.
Undergraduate Student Intern